In the examples given here and here, our cross-form logic depended on data with a known location. In the first case, we knew exactly which event, form, and item to turn to in order to retrieve participate sex and date of birth. In the second case, each of our event dates marked the start of a unique, one-time event, so “finding their address” within the database was a straightforward process.

Now where did I leave that item value?
But what happens when we need to reference data with an indeterminate location, supposing that it even exists? In these cases, we may need to walk around a remote neighborhood, comparing building shapes and sizes, before we find what we’re looking for.
Consider a study that requires drug cessation if a certain adverse event recurs within 90 days. For an Alzheimer’s study, that adverse event may be detection of ARIA (amyloid-related imaging abnormalities) on an MRI scan. Suppose that a second presentation of ARIA within 90 days of the first means that the participant must discontinue study drug. What better occasion could there be for “checking the records” than while reporting a new ARIA? Checking the records here means:
- retrieving the start dates of any previous AE whose report indicated ARIA
- calculating the days’ difference between the most recent of the dates above with the new ARIA presentation date
- showing an alert if that difference is less than 91 days
It’s hardly complex, but a busy CRC working with dozens of participants in multiple trials may forget to follow the process. Cross-form logic, on the other hand, never forgets. The screenshots below depict the result of a third AE report for a single participant. Of the two previous reports, the first indicated detection of ARIA on 1-Nov-2018. Because the newest ARIA, on 24-Jan-2019, falls within 90 days of the prior one, the form displays instructions to discontinue study drug.
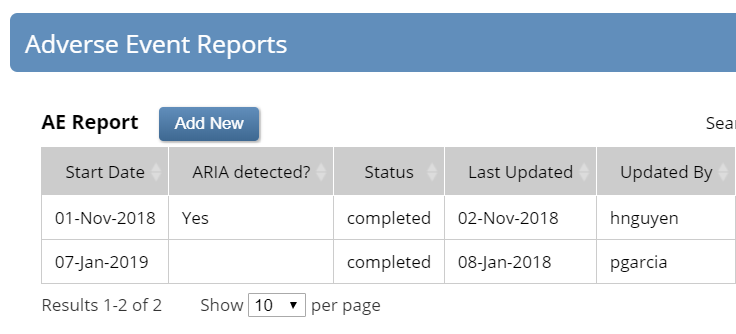
The AE log for this participant shows two reports. The first, documenting an AE on 1-Nov-2018, indicates ARIA.
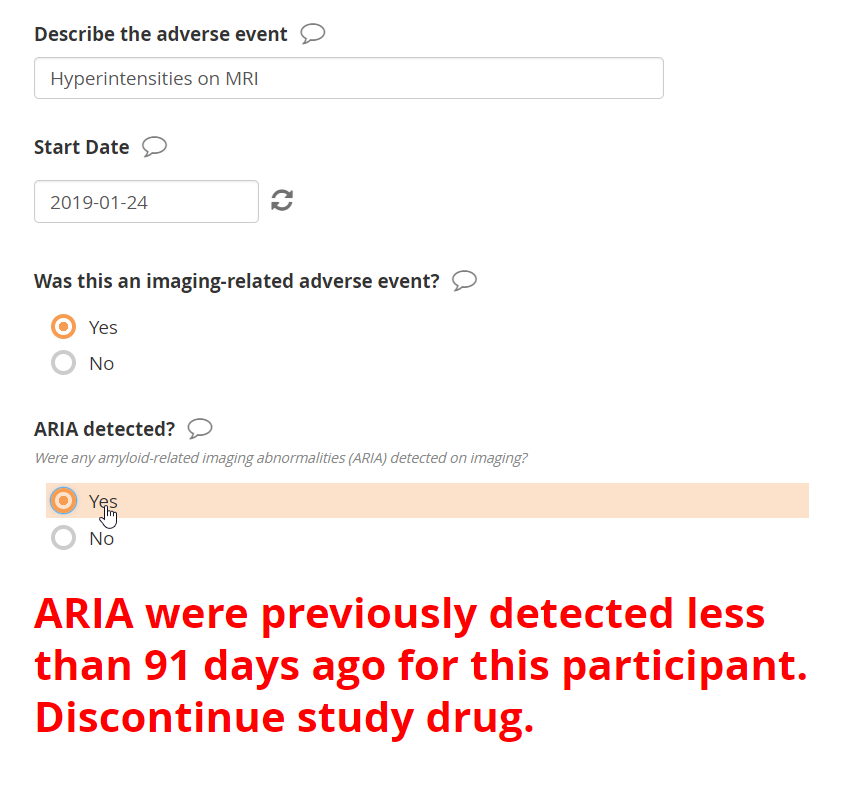
Here the CRC is reporting a new presentation of ARIA, on 24-Jan-2019. That date is fewer than 91 days after the previous ARIA of 1-Nov-2018. As a result, the form displays the relevant instructions from the protocol.
Few questions are too complex for cross-form logic to answer and act upon. If you can state a rule in logical or mathematical terms, you can most likely implement it using a straightforward expression, no matter how many other forms you need to reference. The OpenDataKit library of XPath functions offers a wealth of tools you can combine to create smart, versatile forms that collaborate with researchers. So don’t let your innovation stop with drug development or study design: carry it through to your forms!
