In an ideal clinical study, every lab sample would, within minutes of collection, find its way to a central lab whose equipment was forever up-to-date, whose validations were always fresh, and whose inner workings were transparent to the data manager. But clinical trials aren’t conducted in an ideal world. More often than not, data managers and local lab managers share an ongoing responsibility to document equipment features and report on results collected on a variety of instruments, all calibrated differently. The challenges associated with this process are familiar. Equipment changes. Validations expire. And one lab’s “normal” may be another lab’s “low.”
The task of keeping labs up to date for many data managers is akin to keeping dozens of centrifuges spinning at the same rate, all at the same time. Collecting lab reference ranges from one lab for one analyte may be straightforward, but when the process is multiplied across dozens of analytes and sometimes hundreds of sites, your study can be exposed to significant time delays and human error. Success in this task, like most, hinges on clear expectations and guidance. Here is where good data managers shine. By providing sites with explicit instructions, a deadline, and tools to boost completeness and accuracy, data managers can make the collection of reference ranges a lot less painful and time-consuming.
Anatomy of a Lab Reference Range
Ranges are always defined by either:
- a standard applied to all labs contributing data to a study (“textbook ranges”), or
- the individual lab
Often, the difference between the two is minimal, so adopting the textbook range can save time and administrative burden. For measures that are critical to analysis, though, using a textbook range may not be suitable. In that case, each local lab manager (or the site coordinator representing that lab) must communicate to the study’s data manager their “in house” range for all analytes measured in the study. In both cases, a range is not complete unless it specifies
- the name of analyte
- the unit of measure
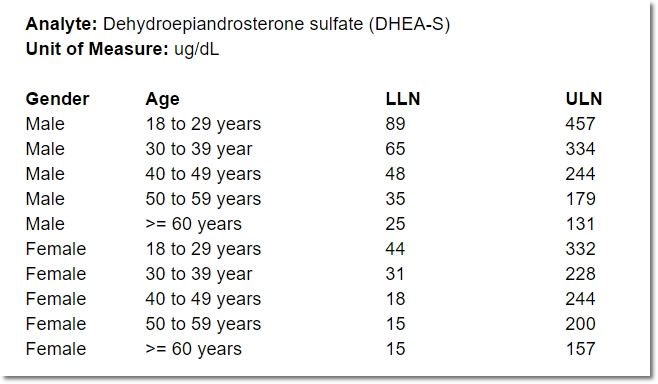
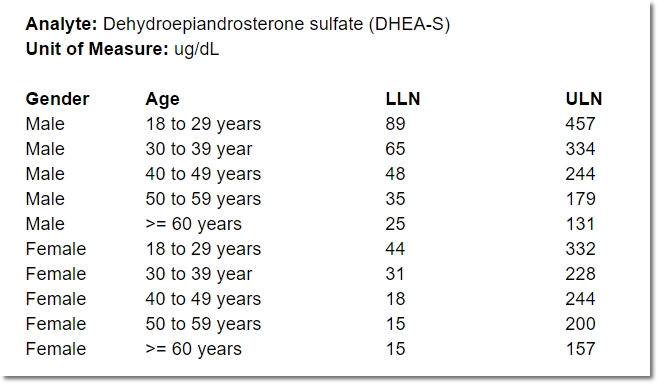
- the lower limit of normal, by gender and age
- the upper limit of normal, by gender and age
Even for one analyte, the normal range for a 25-year-old female may differ from that of a 50-year-old female, or a 25-year-old male. Consequently, specifying a range for an analyte often means specifying a number of “sub-ranges” that, taken together, associate a normal range for every possible patient. For example:
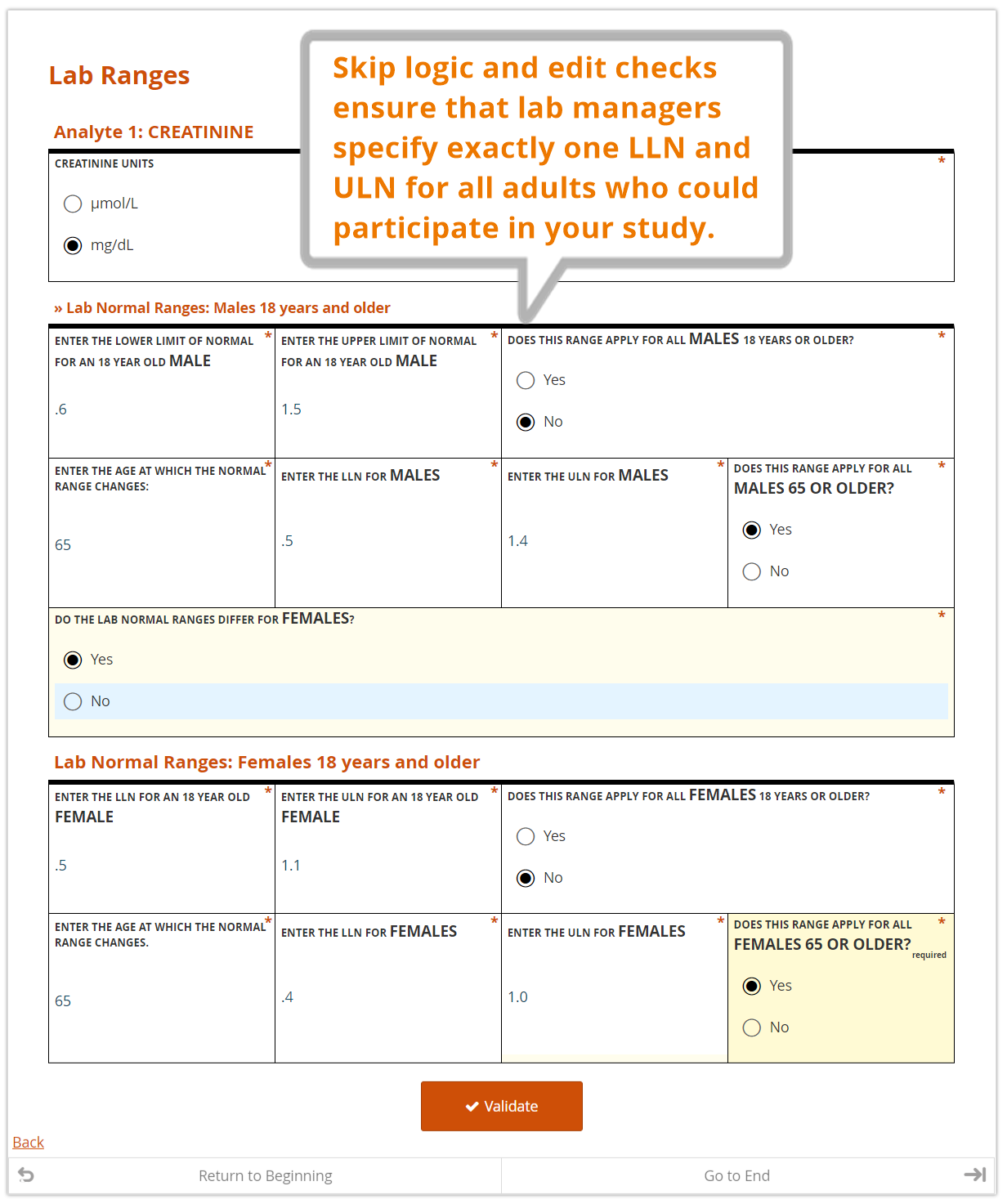
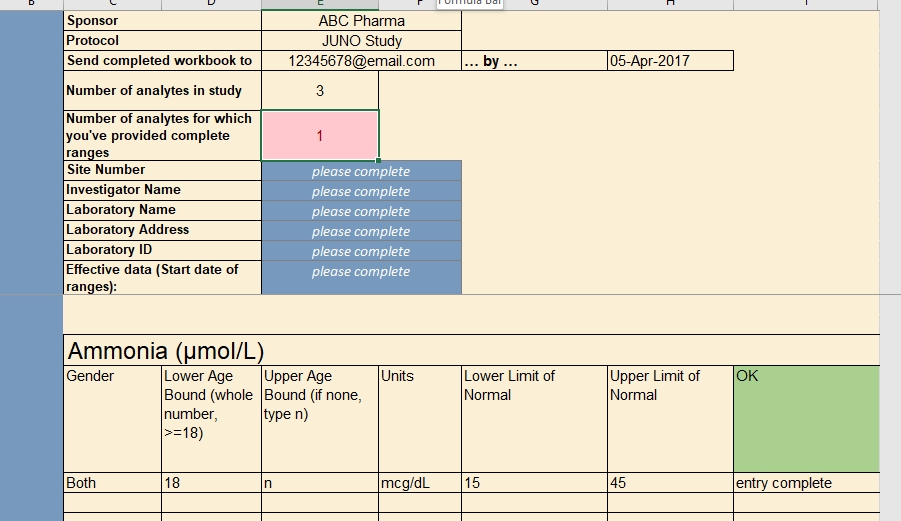
In the course of providing comprehensive ranges for dozens of analytes, it’s easy for a lab representative to overlook (or duplicate) an age or gender inadvertently. A well designed, dynamic form for capturing these requirements can help ensure exactly one range is provided for any given individual.
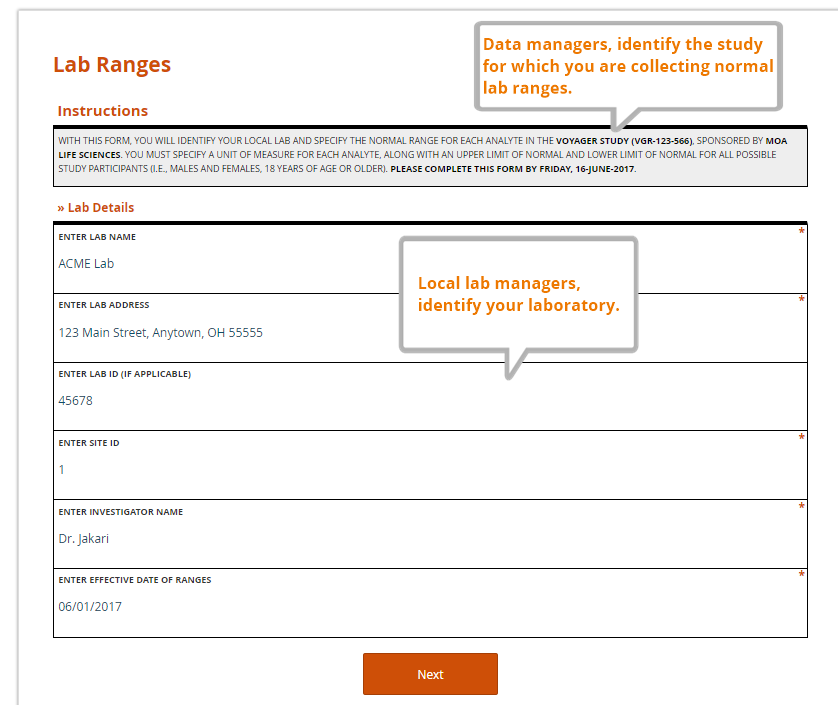
Anatomy of a Lab Reference Range Collection Form
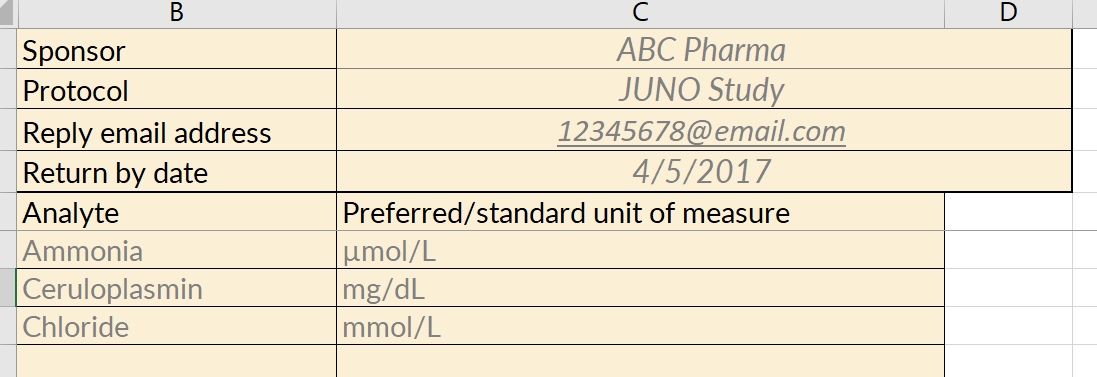
Just as a value without a unit of measure is meaningless, so too is a local lab range that is not tied to a particular lab. Along with their ranges for each study analyte, labs should also provide a set of identifying information. The data manager, as part of her request to provide the ranges and lab information, should also specify the study for which the ranges are being collected. A complete lab reference range collection form includes all of these components.
Specified by the data manager
- the name of the sponsor and study (avoid abbreviations or paraphrases)
- which analytes are included in the study, and therefore require ranges from the lab
- where the lab representative must send the completed file
- a deadline for completing the file
Entered by the lab representative
- the name, address, and applicable ID numbers (e.g. CLF, or core laboratory facility, number) of their lab
- the name of the Principal Investigator for the site and study it serves
- the effective date of the ranges to be provided
- the LLN and ULN for each analyte, by gender and age
Tools You Can Use
For users of OpenClinica, we’ve designed a form template that can be used as a reference range collection form, which includes the components listed above. Would you like to use a customized version of this form in your study? Contact your client support lead.
Staying Current
Regardless of how labs communicate their reference ranges, it’s essential that the communication is ongoing. Changes in equipment or clinical guidelines often occasion changes to upper and lower limits of normal. That’s why an effective date must be documented for all ranges. Good data managers encourage sites to communicate any such changes promptly. Great data managers give them the reminders, and tools, to do so.
We welcome your input on the workbook above, just as we do on our data management metrics calculator. Please let us know what you find most valuable.