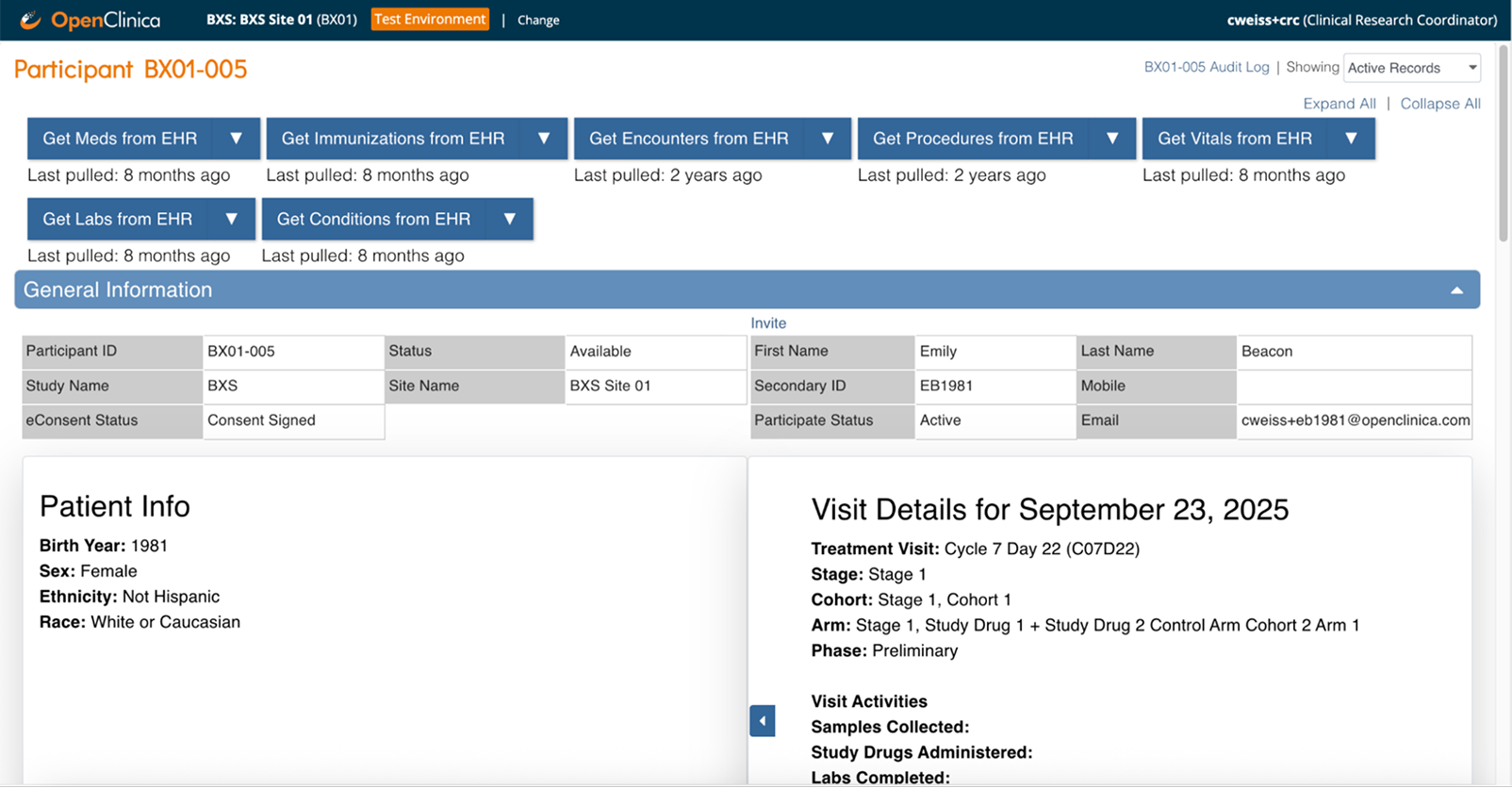
Source data from your EHR without copy-paste.
Automate data flow from patient medical records to your study database, at scale. Eliminate manual transcription, reduce site burden, and speed up source data verification. Backed by 450+ clinical trial sites across major health systems, with the modular flexibility and transparent pricing small to midsize teams need.
Who It’s For

Academic Researchers
Bridge the gap between healthcare and research. Pull clinical data directly into your studies while staying GCP and Part 11 c

Sponsors & CROs
Scale across multiple sites without vendor sprawl. EHR-to-EDC is part of your OpenClinica platform, not another contract to manage.

Sites & Coordinators
Stop retyping vitals, labs, medications, and medical history. Coordinators review and confirm instead of transcribing from scratch.
Benefits
Less site burden, higher satisfaction
Eliminate hours of manual transcription so site staff can focus on patients instead of paperwork—boosting engagement and willingness to participate in future studies.
Superior data quality from day one
EHR source data instills stakeholder confidence and meets national and global compliance benchmarks, with faster identification of safety signals and adverse events.
Faster decisions, reduced time-to-market
Gain early and consistent access to reliable data flowing directly from EHRs to your study forms, enabling quicker decisions.
Cost-effective operations
Reduce data collection costs by 61%—sites using EHR-to-EDC save hours compared to manual data abstraction.
Rapid site activation
FHIR and HL7 standards mean sites become operational in hours, not days or weeks—making it easier for diverse sites to participate.
Future-ready integration
True data interoperability opens doors to novel data sources like medical devices, wearables, and smartphones—expanding therapy development opportunities.
Trusted By: 1500+ sponsors, CROs, and research sites worldwide

















"We save an enormous amount of time and eliminate errors because we can pull laboratory and medication data from our EHR at the click of a button."
Principal Solutions Architect

"The implementation was quick and easy. We received excellent support from the OpenClinica team. They were available and ready to help our strong internal team and we were able to shave a full week off our initial projection."
Project Director, Research/Executive

"We can't recommend the OpenClinica app and team enough, and we are thankful our collaboration continues with other high visibility projects!"
IT User
How it Works
EHR-to-EDC creates a secure bridge between healthcare and research. Built on industry standards (FHIR, HL7), it automates source data acquisition from patient medical record systems to your study database. The result: rigorous GCP and Part 11-compliant data capture at scale, with the transparent pricing and dedicated support small to midsize teams need.
Map your data elements
Identify which clinical data elements (vitals, labs, medications, medical history) need to flow from your EHR into your study CRFs
Connect securely
Establish a secure, standards-based connection between any EHR and OpenClinica EDC using FHIR or HL7 protocols
Automate data flow
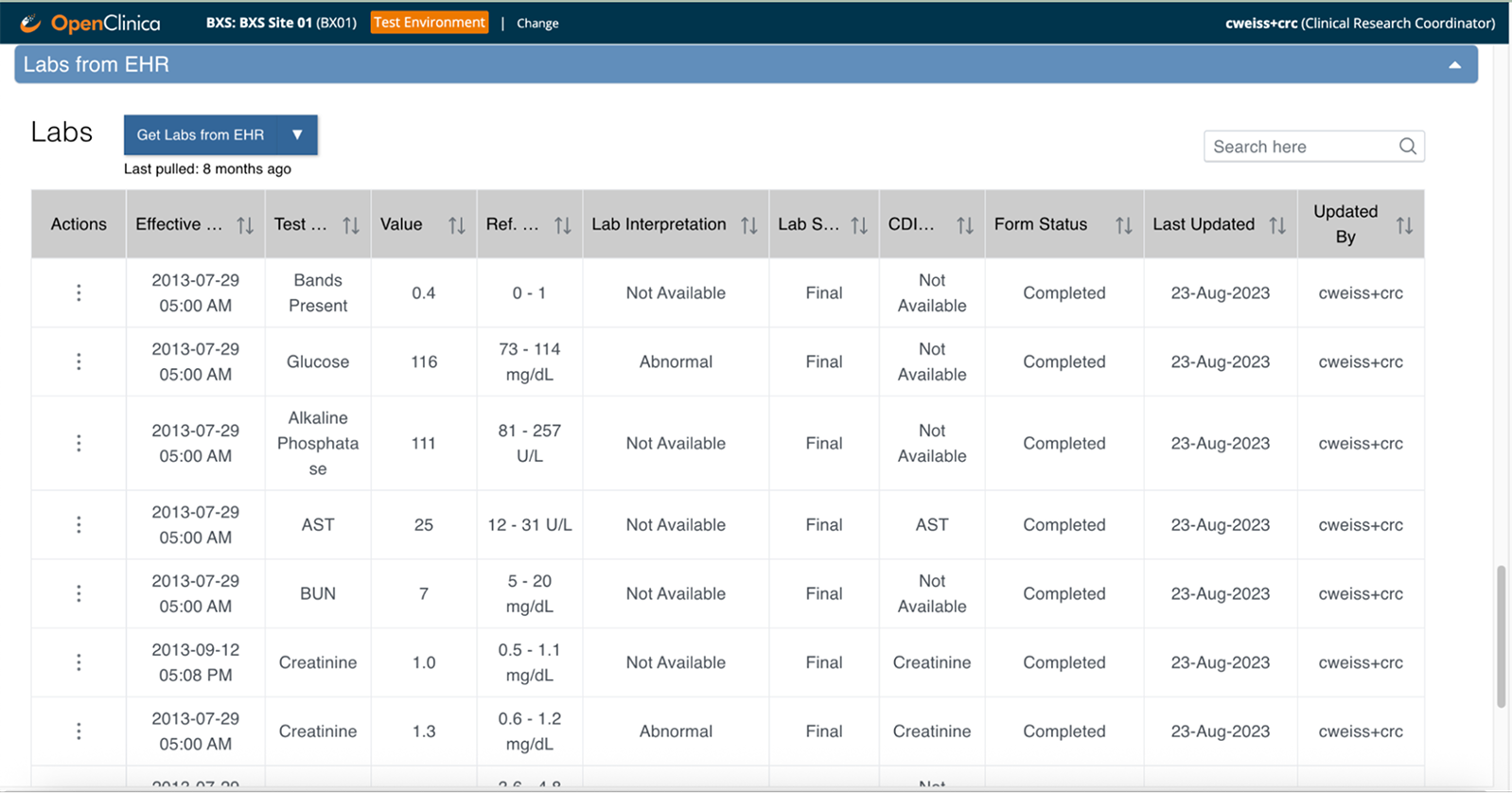
Clinical data flows automatically from your EHR to your study forms—coordinators review and confirm instead of manual transcription
Monitor with confidence
Source data verification happens in real time—monitors verify against the actual EHR source with complete audit trails
Map your data elements
Identify which clinical data elements (vitals, labs, medications, medical history) need to flow from your EHR into your study CRFs

Map your data elements
Connect securely
Establish a secure, standards-based connection between any EHR and OpenClinica EDC using FHIR or HL7 protocols

Connect securely
Automate data flow
Clinical data flows automatically from your EHR to your study forms—coordinators review and confirm instead of manual transcription

Automate data flow
Monitor with confidence
Source data verification happens in real time—monitors verify against the actual EHR source with complete audit trails

Monitor with confidence
Ready to bridge healthcare and research?
Ideal For
- Small to midsize sponsors and CROs
- Academic and health system-based research
- Multi-site studies across health system
- Rigorous GCP and Part 11-compliant trials
- Studies requiring extensive medical history or lab data
- Real-world evidence (RWE) studies
Features
- Standards-based interoperability (FHIR, HL7), not custom integrations
- Secure bridge between EHRs and EDC systems
- Support for major EHR systems (Epic, Cerner, and more)
- Network of 450+ clinical trial sites across major health systems
- Automated source data acquisition at scale
- Real-time data validation and coordinator review workflows
See more
- GCP and 21 CFR Part 11 compliant
- Complete audit trails linking EDC entries to EHR source
- Modular design—works standalone or with OpenClinica EDC
- Transparent, predictable pricing
- 24/5 support included
See How It Works
Ready to eliminate manual transcription?
Share your site network details with us. We’ll discuss how EHR-to-EDC can automate data flow across your sites, saving coordinators hours while improving data quality.